Bonerfried : December 12, 2008

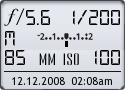
Image Data
File Name: 50D_01765
Model: Canon EOS 50D
Lens: Canon EF-S 17-85mm F4-F5.6 IS USM
Date: 12.12.08 2:08am
Focal Length: 85mm (136mm)
Shutter: 1/200 s
F-Stop: F5.6
ISO: 100
Program: Manual
Metering Mode: Evaluative
Flash: Off
Focus Mode: Manual focus
File Name: 50D_01765
Model: Canon EOS 50D
Lens: Canon EF-S 17-85mm F4-F5.6 IS USM
Date: 12.12.08 2:08am
Focal Length: 85mm (136mm)
Shutter: 1/200 s
F-Stop: F5.6
ISO: 100
Program: Manual
Metering Mode: Evaluative
Flash: Off
Focus Mode: Manual focus
Brass has likely been known to humans since prehistoric times, even before zinc itself was discovered. It was produced by melting copper together with calamine, a zinc ore. In the German village of Breinigerberg, an ancient Roman settlement was discovered where a calamine ore mine existed. During the melting process, the zinc is extracted from the calamine and mixes with the copper. Pure zinc, on the other hand, has too low a boiling point to have been produced by ancient metalworking techniques. The many references to 'brass' appearing throughout the King James Bible are thought to signify another bronze alloy, or copper, rather than the strict modern definition of 'brass'.
Today almost 90% of all brass alloys are recycled. Because brass is nonmagnetic, it can be separated from ferrous scrap by passing the scrap near a powerful magnet. Brass scrap is collected and transported to the foundry where it is melted and recast into billets. Billets are heated and extruded into the desired form and size.
Today almost 90% of all brass alloys are recycled. Because brass is nonmagnetic, it can be separated from ferrous scrap by passing the scrap near a powerful magnet. Brass scrap is collected and transported to the foundry where it is melted and recast into billets. Billets are heated and extruded into the desired form and size.












 Subscribe
Subscribe


