Buhblay : November 18, 2008

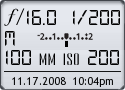
Image Data
File Name: 20D_68255
Model: Canon EOS 20D
Lens: Canon EF 100mm F/2.8 USM Macro
Date: 11.17.08 10:04pm
Focal Length: 100mm (160mm)
Shutter: 1/200 s
F-Stop: F16
ISO: 200
Program: Manual
Metering Mode: Evaluative
Focus Mode: Manual focus
File Name: 20D_68255
Model: Canon EOS 20D
Lens: Canon EF 100mm F/2.8 USM Macro
Date: 11.17.08 10:04pm
Focal Length: 100mm (160mm)
Shutter: 1/200 s
F-Stop: F16
ISO: 200
Program: Manual
Metering Mode: Evaluative
Focus Mode: Manual focus
A bubble can exist because the surface layer of a liquid has a certain surface tension, which causes the layer to behave somewhat like an elastic sheet. However, a bubble made with a pure liquid alone is not stable and a dissolved surfactant such as soap is needed to stabilize a bubble. A common misconception is that soap increases the water's surface tension. Actually soap does the opposite, decreasing it to approximately one third the surface tension of pure water. Soap does not strengthen bubbles, it stabilizes them, via an action known as the Marangoni effect. As the soap film stretches, the surface concentration of soap decreases, which causes the surface tension to increase. Thus, soap selectively strengthens the weakest parts of the bubble and tends to prevent them from stretching further. In addition, the soap reduces evaporation so the bubbles last longer, although this effect is relatively small.
Comments (0)
Colin
11.18.08 12:49pm
Will this be on the quiz next week?
Jasey Michelle 11.18.08 10:50pm
Yes! And I'll release the hounds if I catch you cheating again!
Will this be on the quiz next week?
Jasey Michelle 11.18.08 10:50pm
Yes! And I'll release the hounds if I catch you cheating again!












 Subscribe
Subscribe


