Forked Flamage : January 21, 2008

Image Data
File Name: 20D_13652
Model: Canon EOS 20D
Lens: Canon EF-S 17-85mm F4-F5.6 IS USM
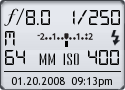
Date: 01.20.08 9:13pm
Focal Length: 64mm (102mm)
Shutter: 1/250 s
F-Stop: F8
ISO: 400
Program: Manual
Metering Mode: Evaluative
Flash: On
Flash Bias: -1.67 EV
Flash Details: External flash, E-TTL
Focus Mode: Manual focus
File Name: 20D_13652
Model: Canon EOS 20D
Lens: Canon EF-S 17-85mm F4-F5.6 IS USM
Date: 01.20.08 9:13pm
Focal Length: 64mm (102mm)
Shutter: 1/250 s
F-Stop: F8
ISO: 400
Program: Manual
Metering Mode: Evaluative
Flash: On
Flash Bias: -1.67 EV
Flash Details: External flash, E-TTL
Focus Mode: Manual focus
The falling matches are frozen with the flash, while the flame is exposed based on the shutter speed.
I sure miss the "Strike Anywhere" matches with the white phosphorus tip. You could light those things with anything! Your zipper, your fingernail, your teeth, your buddies crusty forehead. I guess it was a safety issue, so they stopped selling them in stores ;). I've only seen them online through specialty sites now.
When a safety match is struck on the boxes striking surface, the friction caused by the glass powder striking surface produces enough heat to turn a very small amount of the red phosphorus (on the box) into white phosphorus, which catches fire in air. This small amount of heat is enough to start a chemical reaction that uses the oxidizing agent to produce oxygen gas. The heat and oxygen gas then cause the sulfur to burst into flame, which then catches the wood of the match on fire.
I sure miss the "Strike Anywhere" matches with the white phosphorus tip. You could light those things with anything! Your zipper, your fingernail, your teeth, your buddies crusty forehead. I guess it was a safety issue, so they stopped selling them in stores ;). I've only seen them online through specialty sites now.
When a safety match is struck on the boxes striking surface, the friction caused by the glass powder striking surface produces enough heat to turn a very small amount of the red phosphorus (on the box) into white phosphorus, which catches fire in air. This small amount of heat is enough to start a chemical reaction that uses the oxidizing agent to produce oxygen gas. The heat and oxygen gas then cause the sulfur to burst into flame, which then catches the wood of the match on fire.
Comments (0)
Alyssa Schultz
01.21.08 1:07pm
Wow that's interesting. Now that you're doing this i really do learn something new everyday! Thanks J.... and great photos!
Laurie Bergren 01.28.08 9:06pm
ooh, i LOVE this one (and the explanation)
Wow that's interesting. Now that you're doing this i really do learn something new everyday! Thanks J.... and great photos!
Laurie Bergren 01.28.08 9:06pm
ooh, i LOVE this one (and the explanation)












 Subscribe
Subscribe


