Dihydrogen Monoxide : July 30, 2008

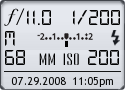
Image Data
File Name: 20D_39350
Model: Canon EOS 20D
Lens: Canon EF-S 17-85mm F4-F5.6 IS USM
Date: 07.29.08 11:05pm
Focal Length: 68mm (109mm)
Shutter: 1/200 s
F-Stop: F11
ISO: 200
Program: Manual
Metering Mode: Evaluative
Flash: On
Flash Details: Manual
Focus Mode: Manual focus
File Name: 20D_39350
Model: Canon EOS 20D
Lens: Canon EF-S 17-85mm F4-F5.6 IS USM
Date: 07.29.08 11:05pm
Focal Length: 68mm (109mm)
Shutter: 1/200 s
F-Stop: F11
ISO: 200
Program: Manual
Metering Mode: Evaluative
Flash: On
Flash Details: Manual
Focus Mode: Manual focus
Dihydrogen monoxide:
Despite the danger, dihydrogen monoxide is often used:
- Is called "hydroxyl acid", the substance is the major component of acid rain.
- Contributes to the "greenhouse effect".
- May cause severe burns.
- Contributes to the erosion of our natural landscape.
- Accelerates corrosion and rusting of many metals.
- May cause electrical failures and decreased effectiveness of automobile brakes.
- Has been found in excised tumors of terminal cancer patients.
Despite the danger, dihydrogen monoxide is often used:
- As an industrial solvent and coolant.
- In nuclear power plants.
- In the production of styrofoam.
- As a fire retardant.
- In many forms of cruel animal research.
- In the distribution of pesticides. Even after washing, produce remains contaminated by this chemical.
- As an additive in certain "junk-foods" and other food products.












 Subscribe
Subscribe


